Tuberculosis is a common and often deadly infectious disease caused by mycobacteria, mainly Mycobacterium tuberculosis. Tuberculosis usually attacks the lungs (as pulmonary TB) but can also affect the central nervous system, the lymphatic system, the circulatory system, the genitourinary system, the gastrointestinal system, bones, joints, and even the skin. Other mycobacteria such as Mycobacterium bovis, Mycobacterium africanum, Mycobacterium canetti, and Mycobacterium microti also cause tuberculosis, but these species are less common.The typical symptoms of tuberculosis are a chronic cough with blood-tinged sputum, fever, night sweats, and weight loss. Infection of other organs causes a wide range of symptoms. The diagnosis relies on radiology (commonly chest X-rays), a tuberculin skin test, blood tests, as well as microscopic examination and microbiological culture of bodily fluids. Tuberculosis treatment is difficult and requires long courses of multiple antibiotics.
Antibiotic resistance is a growing problem in (extensively) multi-drug-resistant tuberculosis. Prevention relies on screening programs and vaccination, usually with Bacillus Calmette-Guérin (BCG vaccine).
Tuberculosis is spread through the air, when people who have the disease cough, sneeze, or spit. One third of the world's current population have been infected with M. tuberculosis, and new infections occur at a rate of one per second. However, most of these cases will not develop the full-blown disease; asymptomatic, latent infection is most common.
In addition, a rising number of people in the developed world are contracting tuberculosis because their immune systems are compromised by immunosuppressive drugs, substance abuse, or AIDS.Other names
In the past, tuberculosis has been called consumption, because it seemed to consume people from within, with a bloody cough, fever, pallor, and long relentless wasting. Other names included phthisis (Greek for consumption) and phthisis pulmonalis; scrofula (in adults), affecting the lymphatic system and resulting in swollen neck glands; tabes mesenterica, TB of the abdomen and lupus vulgaris, TB of the skin; wasting disease; white plague, because sufferers appear markedly pale; king's evil, because it was believed that a king's touch would heal scrofula; and Pott's disease, or gibbus of the spine and joints.
Symptoms
When the disease becomes active, 75% of the cases are pulmonary TB. Symptoms include chest pain, coughing up blood, and a productive, prolonged cough for more than three weeks. Systemic symptoms include fever, chills, night sweats, appetite loss, weight loss, pallor, and often a tendency to fatigue very easily.
In the other 25% of active cases, the infection moves from the lungs, causing other kinds of TB. This occurs more commonly in immunosuppressed persons and young children. Extrapulmonary infection sites include the pleura, the central nervous system in meningitis, the lymphatic system in scrofula of the neck, the genitourinary system in urogenital tuberculosis, and bones and joints in Pott's disease of the spine. An especially serious form is disseminated TB, more commonly known as miliary tuberculosis. Although extrapulmonary TB is not contagious, it may co-exist with pulmonary TB, which is contagious.
Bacterial species
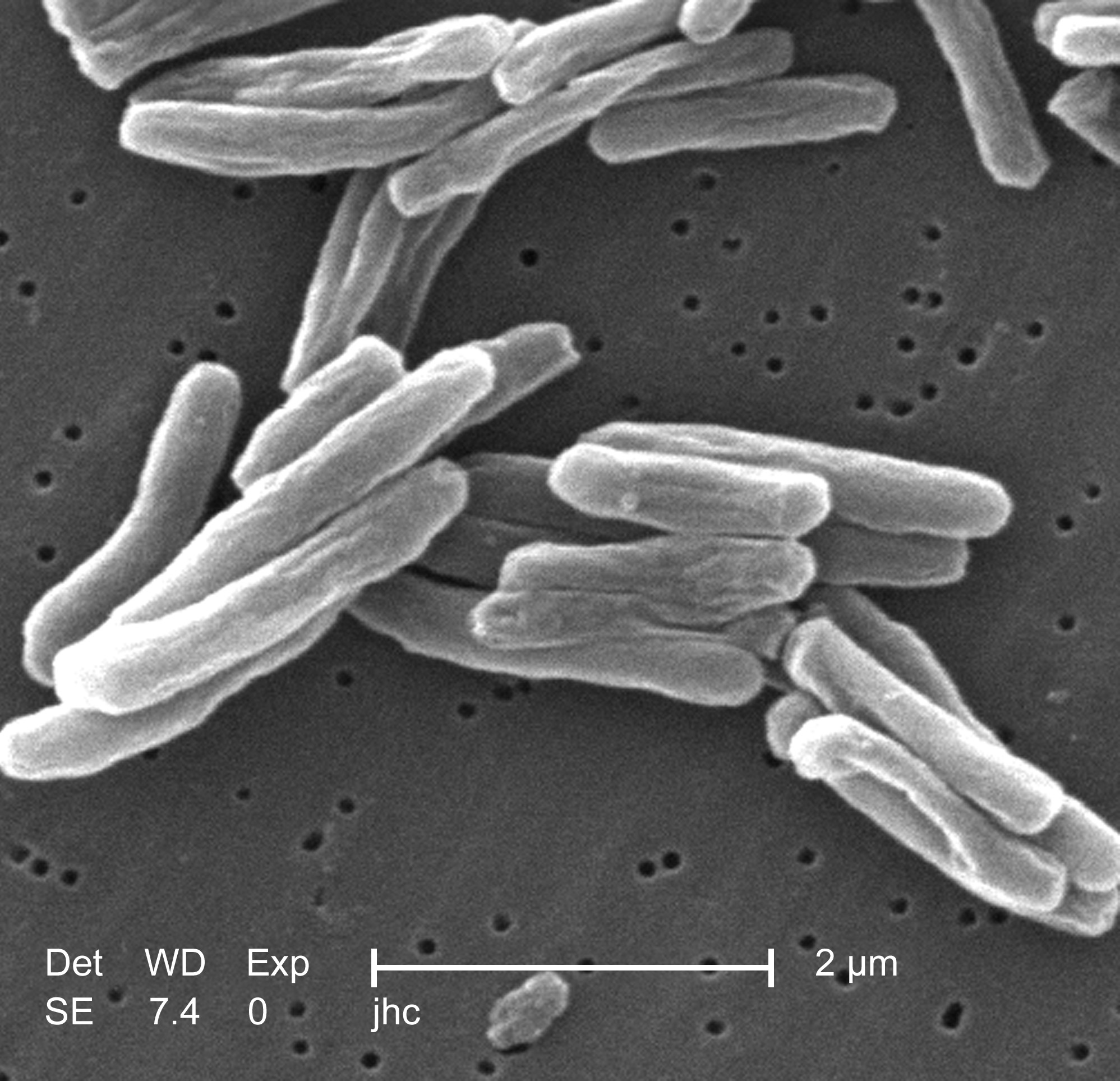
The primary cause of TB, Mycobacterium tuberculosis, is an aerobic bacterium that divides every 16 to 20 hours, an extremely slow rate compared with other bacteria, which usually divide in less than an hour. (For example, one of the fastest-growing bacteria is a strain of E. coli that can divide roughly every 20 minutes.) Since MTB has a cell wall but lacks a phospholipid outer membrane, it is classified as a Gram-positive bacterium. However, if a Gram stain is performed, MTB either stains very weakly Gram-positive or does not retain dye due to the high lipid & mycolic acid content of its cell wall. MTB is a small rod-like bacillus that can withstand weak disinfectants and survive in a dry state for weeks. In nature, the bacterium can grow only within the cells of a host organism, but M. tuberculosis can be cultured in vitro.
Using histological stains on expectorate samples from phlegm (also called sputum), scientists can identify MTB under a regular microscope. Since MTB retains certain stains after being treated with acidic solution, it is classified as an acid-fast bacillus (AFB). The most common acid-fast staining technique, the Ziehl-Neelsen stain, dyes AFBs a bright red that stands out clearly against a blue background. Other ways to visualize AFBs include an auramine-rhodamine stain and fluorescent microscopy.
The M. tuberculosis complex includes three other TB-causing mycobacteria: M. bovis, M. africanum and M. microti.
M. bovis was once a common cause of tuberculosis, but the introduction of pasteurized milk has largely eliminated this as a public health problem in developed countries. M. microti is mostly seen in immunodeficient people, although it is possible that the prevalence of this pathogen has been underestimated.
Other known pathogenic mycobacteria include Mycobacterium leprae, Mycobacterium avium and M. kansasii. The last two are part of the nontuberculous mycobacteria (NTM) group. Nontuberculous mycobacteria cause neither TB nor leprosy, but they do cause pulmonary diseases resembling TB.
Transmission
When people suffering from active pulmonary TB cough, sneeze, speak, or spit, they expel infectious aerosol droplets 0.5 to 5 µm in diameter. A single sneeze can release up to 40,000 droplets. Each one of these droplets may transmit the disease, since the infectious dose of tuberculosis is very low and the inhalation of just a single bacterium can cause a new infection.
People with prolonged, frequent, or intense contact are at particularly high risk of becoming infected, with an estimated 22% infection rate. A person with active but untreated tuberculosis can infect 10–15 other people per year. Others at risk include people in areas where TB is common, people who inject drugs using unsanitary needles, residents and employees of high-risk congregate settings, medically under-served and low-income populations, high-risk racial or ethnic minority populations, children exposed to adults in high-risk categories, patients immunocompromised by conditions such as HIV/AIDS, people who take immunosuppressant drugs, and health care workers serving these high-risk clients.
Transmission can only occur from people with active — not latent — TB. The probability of transmission from one person to another depends upon the number of infectious droplets expelled by a carrier, the effectiveness of ventilation, the duration of exposure, and the virulence of the M. tuberculosis strain. The chain of transmission can, therefore, be broken by isolating patients with active disease and starting effective anti-tuberculous therapy. After two weeks of such treatment, people with non-resistant active TB generally cease to be contagious. If someone does become infected, then it will take at least 21 days, or three to four weeks, before the newly infected person can transmit the disease to others. TB can also be transmitted by eating meat infected with TB. Mycobacterium bovis causes TB in cattle.
Pathogenesis
About 90% of those infected with Mycobacterium tuberculosis have asymptomatic, latent TB infection (sometimes called LTBI), with only a 10% lifetime chance that a latent infection will progress to TB disease. However, if untreated, the death rate for these active TB cases is more than 50%.
TB infection begins when the mycobacteria reach the pulmonary alveoli, where they invade and replicate within the endosomes of alveolar macrophages. The primary site of infection in the lungs is called the Ghon focus, and is generally located in either the upper part of the lower lobe, or the lower part of the upper lobe. Bacteria are picked up by dendritic cells, which do not allow replication, although these cells can transport the bacilli to local (mediastinal) lymph nodes. Further spread is through the bloodstream to other tissues and organs where secondary TB lesions can develop in other parts of the lung (particularly the apex of the upper lobes), peripheral lymph nodes, kidneys, brain, and bone. All parts of the body can be affected by the disease, though it rarely affects the heart, skeletal muscles, pancreas and thyroid.
Tuberculosis is classified as one of the granulomatous inflammatory conditions. Macrophages, T lymphocytes, B lymphocytes and fibroblasts are among the cells that aggregate to form a granuloma, with lymphocytes surrounding the infected macrophages. The granuloma functions not only to prevent dissemination of the mycobacteria, but also provides a local environment for communication of cells of the immune system. Within the granuloma, T lymphocytes (CD4+) secrete cytokines such as interferon gamma, which activates macrophages to destroy the bacteria with which they are infected. T lymphocytes (CD8+) can also directly kill infected cells.
Importantly, bacteria are not always eliminated within the granuloma, but can become dormant, resulting in a latent infection. Another feature of the granulomas of human tuberculosis is the development of cell death, also called necrosis, in the center of tubercles. To the naked eye this has the texture of soft white cheese and was termed caseous necrosis.
If TB bacteria gain entry to the bloodstream from an area of damaged tissue they spread through the body and set up many foci of infection, all appearing as tiny white tubercles in the tissues. This severe form of TB disease is most common in infants and the elderly and is called miliary tuberculosis. Patients with this disseminated TB have a fatality rate of approximately 20%, even with intensive treatment.
In many patients the infection waxes and wanes. Tissue destruction and necrosis are balanced by healing and fibrosis. Affected tissue is replaced by scarring and cavities filled with cheese-like white necrotic material. During active disease, some of these cavities are joined to the air passages bronchi and this material can be coughed up. It contains living bacteria and can therefore pass on infection. Treatment with appropriate antibiotics kills bacteria and allows healing to take place. Upon cure, affected areas are eventually replaced by scar tissue.
Diagnosis

Tuberculosis is diagnosed definitively by identifying the causative organism (Mycobacterium tuberculosis) in a clinical sample (for example, sputum or pus). When this is not possible, a probable diagnosis may be made using imaging (X-rays or scans) or a tuberculin skin test.
The main problem with tuberculosis diagnosis is the difficulty in culturing this slow-growing organism in the laboratory (it may take 4 to 12 weeks for blood or sputum culture). A complete medical evaluation for TB must include a medical history, a physical examination, a chest X-ray, microbiological smears and cultures. It may also include a tuberculin skin test, a serological test. The interpretation of the tuberculin skin test depends upon the person's risk factors for infection and progression to TB disease, such as exposure to other cases of TB or immunosuppression.
Currently, latent infection is diagnosed in a non-immunized person by a tuberculin skin test, which yields a delayed hypersensitivity type response to an extract made from M. tuberculosis. Those immunized for TB or with past-cleared infection will respond with delayed hypersensitivity parallel to those currently in a state of infection, so the test must be used with caution, particularly with regard to persons from countries where TB immunization is common. Tuberculin tests have the disadvantage in that they may produce false negatives, especially when the patient is co-morbid with sarcoidosis, Hodgkins lymphoma, malnutrition, or most notably active tuberculosis disease. New TB tests are being developed that offer the hope of cheap, fast and more accurate TB testing. These use polymerase chain reaction detection of bacterial DNA and antibody assays to detect the release of interferon gamma in response to mycobacteria. These tests are not affected by immunization, so generate fewer false positive results. The development of a rapid and inexpensive diagnosis would be particularly valuable in the developing world.
Progression
Progression from TB infection to TB disease occurs when the TB bacilli overcome the immune system defenses and begin to multiply. In primary TB disease—1–5% of cases—this occurs soon after infection. However, in the majority of cases, a latent infection occurs that has no obvious symptoms. These dormant bacilli can produce tuberculosis in 2–23% of these latent cases, often many years after infection. The risk of reactivation increases with immunosuppression, such as that caused by infection with HIV. In patients co-infected with M. tuberculosis and HIV, the risk of reactivation increases to 10% per year.
Patients with diabetes mellitus are at increased risk of contracting tuberculosis, and they have a poorer response to treatment, possibly due to poorer drug absorption
Other conditions that increase risk include IV drug abuse; recent TB infection or a history of inadequately treated TB; chest X-ray suggestive of previous TB, showing fibrotic lesions and nodules; silicosis; prolonged corticosteroid therapy and other immunosuppressive therapy; head and neck cancers; hematologic and reticuloendothelial diseases, such as leukemia and Hodgkin's disease; end-stage kidney disease; intestinal bypass or gastrectomy; chronic malabsorption syndromes; vitamin D deficiency; and low body weight.
Twin studies in the 1940s showed that susceptibility to TB was heritable. If one of a pair of twins got TB, then and the other was more likely to get TB if he was identical than if he was not. Since then, specific gene polymorphisms in IL12B have been linked to tuberculosis susceptibility.
Some drugs, including rheumatoid arthritis drugs that work by blocking tumor necrosis factor-alpha (an inflammation-causing cytokine), raise the risk of activating a latent infection due to the importance of this cytokine in the immune defense against TB.
Treatment
Treatment for TB uses antibiotics to kill the bacteria. The two antibiotics most commonly used are rifampicin and isoniazid. However, instead of the short course of antibiotics typically used to cure other bacterial infections, TB requires much longer periods of treatment (around 6 to 12 months) to entirely eliminate mycobacteria from the body. Latent TB treatment usually uses a single antibiotic, while active TB disease is best treated with combinations of several antibiotics, to reduce the risk of the bacteria developing antibiotic resistance. People with latent infections are treated to prevent them from progressing to active TB disease later in life. However, treatment using Rifampin and Pyrazinamide is not risk-free. The Centers for Disease Control and Prevention (CDC) notified healthcare professionals of revised recommendations against the use of rifampin plus pyrazinamide for treatment of latent tuberculosis infection, due to high rates of hospitalization and death from liver injury associated with the combined use of these drugs.
Drug resistant tuberculosis is transmitted in the same way as regular TB. Primary resistance occurs in persons who are infected with a resistant strain of TB. A patient with fully-susceptible TB develops secondary resistance (acquired resistance) during TB therapy because of inadequate treatment, not taking the prescribed regimen appropriately, or using low quality medication. Drug-resistant TB is a public health issue in many developing countries, as treatment is longer and requires more expensive drugs. Multi-drug resistant TB (MDR-TB) is defined as resistance to the two most effective first line TB drugs: rifampicin and isoniazid. Extensively drug-resistant TB (XDR-TB) is also resistant to three or more of the six classes of second-line drugs.
In ancient times, available treatments focused more on dietary parameters. Pliny the Elder described several methods in his Natural History: "wolf's liver taken in thin wine, the lard of a sow that has been fed upon grass, or the flesh of a she-ass taken in broth". While these particular remedies haven't been tested scientifically, it has been demonstrated that malnourished mice receiving a 2% protein diet suffer far higher mortality from tuberculosis than those receiving 20% protein receiving the same infectious challenge dose, and the progressively fatal course of the illness could be reversed by restoring the mice to the normal diet.
Prevention
TB prevention and control takes two parallel approaches. In the first, people with TB and their contacts are identified and then treated. Identification of infections often involves testing high-risk groups for TB. In the second approach, children are vaccinated to protect them from TB. Unfortunately, no vaccine is available that provides reliable protection for adults. However, in tropical areas where the levels of other species of mycobacteria are high, exposure to nontuberculous mycobacteria gives some protection against TB.
The World Health Organization (W.H.O.) declared TB a global health emergency in 1993, and the Stop TB Partnership developed a Global Plan to Stop Tuberculosis that aims to save 14 million lives between 2006 and 2015. Since humans are the only host of Mycobacterium tuberculosis, eradication would be possible: a goal that would be helped greatly by an effective vaccine.
Vaccines
Many countries use Bacillus Calmette-Guérin (BCG) vaccine as part of their TB control programs, especially for infants. According to the W.H.O., this is the most often used vaccine worldwide, with 85% of infants in 172 countries. This was the first vaccine for TB and developed at the Pasteur Institute in France between 1905 and 1921. The protective efficacy of BCG for preventing serious forms of TB (e.g. meningitis) in children is greater than 80%; its protective efficacy for preventing pulmonary TB in adolescents and adults is variable, ranging from 0 to 80%.
In South Africa, the country with the highest prevalence of TB, BCG is given to all children under age three. However, BCG is less effective in areas where mycobacteria are less prevalent; therefore BCG is not given to the entire population in these countries. In the USA, for example, BCG vaccine is not recommended except for people who meet specific criteria:
- Infants or children with negative skin test results who are continually exposed to untreated or ineffectively treated patients or will be continually exposed to multidrug-resistant TB.
- Healthcare workers considered on an individual basis in settings in which a high percentage of MDR-TB patients has been found, transmission of MDR-TB is likely, and TB control precautions have been implemented and were not successful.
BCG provides some protection against severe forms of pediatric TB, but has been shown to be unreliable against adult pulmonary TB, which accounts for most of the disease burden worldwide. Currently, there are more cases of TB on the planet than at any other time in history and most agree there is an urgent need for a newer, more effective vaccine that would prevent all forms of TB—including drug resistant strains—in all age groups and among people with HIV.
Several new vaccines to prevent TB infection are being developed. The first recombinant tuberculosis vaccine entered clinical trials, sponsored by the National Institute of Allergy and Infectious Diseases (NIAID). A 2005 study showed that a DNA TB vaccine given with conventional chemotherapy can accelerate the disappearance of bacteria as well as protect against re-infection in mice; it may take four to five years to be available in humans. Many other strategies are also being used to develop novel vaccines. In order to encourage further discovery, researchers and policymakers are promoting new economic models of vaccine development including prizes, tax incentives and advance market commitments.












No comments:
Post a Comment