Progeria is a condition that resembles premature aging which usually refers specifically to Hutchinson-Gilford Progeria syndrome.
Hutchinson-Gilford Progeria syndrome is an extremely rare condition where symptoms resembling some aspects of aging are manifested at an early age, and few affected children live past age 13. About 1 in 8 million babies are born with this condition. It is a genetic condition, but occurs sporadically and is usually not inherited in families.
Scientists are particularly interested in progeria because it might reveal clues about the normal process of aging.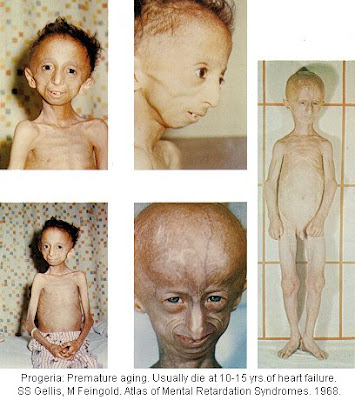
Symptoms
The earliest symptoms include failure to thrive (FTT) and a localized scleroderma-like skin condition. As the child ages past infancy, additional conditions become apparent. Limited growth, alopecia, and a distinctive appearance with small face and jaw and pinched nose all are characteristic of progeria. The people diagnosed with this disease usually have small, fragile bodies like those of elderly people.
Later the condition causes wrinkled skin, atherosclerosis and cardiovascular problems.
Cause
Hutchinson-Gilford Progeria Syndrome (HGPS) is a childhood disorder caused by mutations in one of the major architectural proteins of the cell nucleus.
Unlike most other "accelerated aging diseases" (such as Werner's syndrome, Cockayne's syndrome or xeroderma pigmentosum), progeria is not caused by defective DNA repair. Because these "accelerated aging" diseases display different aspects of aging, but never every aspect, they are often called "segmental progerias".
Diagnosis
Diagnosis is suspected according to signs and symptoms, such as skin changes, abnormal growth, and loss of hair. It can be confirmed through a genetic test.
Treatment
No treatments have been proven effective. Most treatment focuses on reducing complications such as cardiovascular disease, such as heart bypass surgery or low-dose aspirin. Children may also benefit from a high-calorie diet.
Growth hormone treatment has been attempted.
A type of anti-cancer drug, the farnesyltransferase inhibitors (FTIs), have been proposed, but their use has been mostly limited to animal models. A phase II clinical trial using the FTI Lonafarnib began in May 2007.
Prognosis
There is no known cure. Few people with progeria exceed 13 years of age. At least 90% of patients die from complications of atherosclerosis, such as heart attacks or strokes.
Mental development is not affected. The development of symptoms is comparable to aging at a rate six to eight times faster than normal, although certain age-related conditions do not occur. Specifically, patients show no neurodegeneration or cancer predisposition. They do not develop "wear and tear" conditions commonly associated with aging, like cataracts and osteoarthritis.
Epidemiology
One study from the Netherlands has shown an incidence of 1 in 4 million births. Currently, there are 48 known cases in the world. Approximately 100 cases have been formally identified in medical history.
Classical Hutchinson–Gilford Progeria Syndrome is almost never passed on from parent to child. It is usually caused by a new (sporadic) mutation during the early division of the cells in the child. There has been one case in which became evident that a healthy parent can carry the LMNA-mutation that causes progeria in her or his egg- or spermcells. In this case two siblings were born with HGPS. But Hutchinson–Gilford Progeria Syndrome is usually genetically dominant, therefore parents who are healthy will normally not pass it on to their children. Affected children do not live long enough to have children themselves.
However, there are milder cases in which either the gene is not expressed in parents, or a different gene is responsible for a different form of progeria, and healthy parents can pass on their children.
Four families have been identified as having more than one child with the disease.
Research areas
Several discoveries have been made that have led to greater understanding and perhaps eventual treatment.
A 2003 report in Nature said progeria may be a de novo dominant trait. It develops during cell division in a newly conceived child or in the gametes of one of the parents. It is caused by mutations in LMNA (Lamin A protein) gene on chromosome 1; The mutated form of Lamin A is commonly known as progerin. One of the authors, Leslie Gordon, was a physician who didn't know anything about progeria, until her own son, Sam, was diagnosed at 21 months. Gordon and her husband, pediatrician Scott Berns, founded the Progeria Research Foundation.
Lamin A
Nuclear lamina is a protein scaffold on the inner edge of the nucleus that helps organize nuclear processes such as RNA and DNA synthesis.
preLamin A contains a CAAX box at the C-terminus of the protein (where C is a cysteine and A is any aliphatic amino acids). This ensures that the cysteine is farnesylated, and this allows preLamin A to bind membranes, specifically the nuclear membrane. After Prelamin A has been localized to the cell nuclear membrane the C-terminal amino acids, including the farnesylated cysteine, are cleaved off by a specific protease. The resulting protein is now Lamin A, is no longer membrane-bound and carries out functions inside the nucleus.
In HGPS the recognition site that the enzyme requires for cleavage of Prelamin A to Lamin A is mutated. Lamin A cannot be produced and preLamin A builds up on the nuclear membrane, causing a characteristic nuclear blebbing. This results in the premature aging symptoms of progeria, although the mechanism connecting the misshapen nucleus to the symptoms is not known.
A study which compared HGPS patient cells with the skin cells from LMNA young and elderly human subjects found similar defects in the HGPS and elderly cells, including down-regulation of certain nuclear proteins, increased DNA damage and demethylation of histone leading to reduced heterochromatin. Nematodes over their lifespan show progressive lamin changes comparable to HGPS in all cells but neurons and gametes. These studies suggest that lamin A defects contribute to normal aging.
Mouse model of progeria
A mouse model of progeria exists, though in the mouse the LMNA preLamin A is not mutated, but instead ZMPSTE24, the specific protease that is required to remove the C-terminus of preLamin A is missing. Both cases result in the build up of farnesylated preLamin A on the nuclear membrane and in the characteristic nuclear LMNA blebbing. Fong et al use a farnesyl transferase inhibitor (FTI) in this mouse model to inhibit protein farnesylation of preLamin A. Treated mice had greater grip strength, lower likelihood of rib fracture and may live longer than untreated mice.
This method does not directly 'cure' the underlying cause of progeria. This method prevents Prelamin A going to the nucleus in the first place so no preLamin A can build up on the nuclear membrane, but equally there is no production of normal Lamin A in the nucleus. Luckily Lamin A does not appear to be essential, indeed mouse models in which the genes for preLamin A and C are knocked out show no symptoms. This also shows that it is the build up of Prelamin A in the wrong place, rather than the loss of the normal function of Lamin A that causes the disease.
It was hypothesized that part of the reason that treatment with an FFI such as alendronate is inefficient due to prenylation by geranylgeranyltransferase. Since statins inhibit geranylgeranyltransferase, the combination of an FFI and statins was tried, and markedly improved "the aging-like phenotypes of mice deficient in the metalloproteinase Zmpste24, including growth retardation, loss of weight, lipodystrophy, hair loss and bone defects".
History
Progeria was first described in 1886 by Jonathan Hutchinson and also described independently in 1897 by Hastings Gilford. The condition was later named Hutchinson-Gilford Progeria syndrome (HGPS).












No comments:
Post a Comment