The greenhouse effect refers to the change in the thermal equilibrium temperature of a planet or moon by the presence of an atmosphere containing gas that absorbs and emits infrared radiation. Greenhouse gases, which include water vapor, carbon dioxide and methane, warm the atmosphere by efficiently absorbing thermal infrared radiation emitted by the Earth’s surface, by the atmosphere itself, and by clouds. As a result of its warmth, the atmosphere also radiates thermal infrared in all directions, including downward to the Earth’s surface. Thus, greenhouse gases trap heat within the surface-troposphere system. This mechanism is fundamentally different from the mechanism of an actual greenhouse, which instead isolates air inside the structure so that heat is not lost by convection and conduction, as discussed below. The greenhouse effect was discovered by Joseph Fourier in 1824, first reliably experimented on by John Tyndall in the year 1858 and first reported quantitatively by Svante Arrhenius in his 1896 paper.
In the absence of the greenhouse effect and an atmosphere, the Earth's average surface temperature of 14 °C (57 °F) could be as low as −18 °C (−0.4 °F), the black body temperature of the Earth.
Anthropogenic global warming (AGW), a recent warming of the Earth's lower atmosphere as evidenced by the global mean temperature anomaly trend, is believed to be the result of an "enhanced greenhouse effect" mainly due to human-produced increased concentrations of greenhouse gases in the atmosphere and changes in the use of land.
The greenhouse effect is one of several factors which affect the temperature of the Earth. Other positive and negative feedbacks dampen or amplify the greenhouse effect. In our solar system, Mars, Venus, and the moon Titan also exhibit greenhouse effects according to their respective environments. In addition, Titan has an anti-greenhouse effect and Pluto exhibits behavior similar to the anti-greenhouse effect.Basic mechanism
The Earth receives energy from the Sun mostly in the form of visible light. The bulk of this energy is not absorbed by the atmosphere since the atmosphere is transparent to visible light. 50% of the sun's energy reaches the Earth which is absorbed by the surface as heat. Because of its temperature, the Earth's surface radiates energy in infrared range. The Greenhouse gases are not transparent to infrared radiation so they absorb infrared radiation. Infrared radiation is absorbed from all directions and is passed as heat to all gases in the atmosphere. The atmosphere also radiates in the infrared range (because of its temperature, in the same way the Earth's surface does) and does so in all directions. The surface and lower atmosphere are warmed because of the greenhouse gases and makes our life on earth possible.
Detailed explanation
The Earth receives energy from the Sun in the form of radiation. Most of the energy is in visible wavelengths and in infrared wavelengths that are near the visible range (often called "near infrared"). The Earth reflects about 30% of the incoming solar radiation. The remaining 70% is absorbed, warming the land, atmosphere and ocean.
For the Earth's temperature to be in steady state so that the Earth does not rapidly heat or cool, this absorbed solar radiation must be very closely balanced by energy radiated back to space in the infrared wavelengths. Since the intensity of infrared radiation increases with increasing temperature, one can think of the Earth's temperature as being determined by the infrared flux needed to balance the absorbed solar flux. The visible solar radiation mostly heats the surface, not the atmosphere, whereas most of the infrared radiation escaping to space is emitted from the upper atmosphere, not the surface. The infrared photons emitted by the surface are mostly absorbed in the atmosphere by greenhouse gases and clouds and do not escape directly to space.
The reason this warms the surface is most easily understood by starting with a simplified model of a purely radiative greenhouse effect that ignores energy transfer in the atmosphere by convection (sensible heat transport, Sensible heat flux) and by the evaporation and condensation of water vapor (latent heat transport, Latent heat flux). In this purely radiative case, one can think of the atmosphere as emitting infrared radiation both upwards and downwards. The upward infrared flux emitted by the surface must balance not only the absorbed solar flux but also this downward infrared flux emitted by the atmosphere. The surface temperature will rise until it generates thermal radiation equivalent to the sum of the incoming solar and infrared radiation.
A more realistic picture taking into account the convective and latent heat fluxes is somewhat more complex. But the following simple model captures the essence. The starting point is to note that the opacity of the atmosphere to infrared radiation determines the height in the atmosphere from which most of the photons are emitted into space. If the atmosphere is more opaque, the typical photon escaping to space will be emitted from higher in the atmosphere, because one then has to go to higher altitudes to see out to space in the infrared. Since the emission of infrared radiation is a function of temperature, it is the temperature of the atmosphere at this emission level that is effectively determined by the requirement that the emitted flux balance the absorbed solar flux.
But the temperature of the atmosphere generally decreases with height above the surface, at a rate of roughly 6.5 °C per kilometer on average, until one reaches the stratosphere 10–15 km above the surface. (Most infrared photons escaping to space are emitted by the troposphere, the region bounded by the surface and the stratosphere, so we can ignore the stratosphere in this simple picture.) A very simple model, but one that proves to be remarkably useful, involves the assumption that this temperature profile is simply fixed, by the non-radiative energy fluxes. Given the temperature at the emission level of the infrared flux escaping to space, one then computes the surface temperature by increasing temperature at the rate of 6.5 °C per kilometer, the environmental lapse rate, until one reaches the surface. The more opaque the atmosphere, and the higher the emission level of the escaping infrared radiation, the warmer the surface, since one then needs to follow this lapse rate over a larger distance in the vertical. While less intuitive than the purely radiative greenhouse effect, this less familiar radiative-convective picture is the starting point for most discussions of the greenhouse effect in the climate modeling literature.
Greenhouse gases
Quantum mechanics provides the basis for computing the interactions between molecules and radiation. Most of this interaction occurs when the frequency of the radiation closely matches that of the spectral lines of the molecule, determined by the quantization of the modes of vibration and rotation of the molecule. (The electronic excitations are generally not relevant for infrared radiation, as they require energy larger than that in an infrared photon.)
The width of a spectral line is an important element in understanding its importance for the absorption of radiation. In the Earth’s atmosphere these spectral widths are primarily determined by “pressure broadening”, which is the distortion of the spectrum due to the collision with another molecule. Most of the infrared absorption in the atmosphere can be thought of as occurring while two molecules are colliding. The absorption due to a photon interacting with a lone molecule is relatively small. This three-body aspect of the problem, one photon and two molecules, makes direct quantum mechanical computation for molecules of interest more challenging. Careful laboratory spectroscopic measurements, rather than ab initio quantum mechanical computations, provide the basis for most of the radiative transfer calculations used in studies of the atmosphere.
The molecules/atoms that constitute the bulk of the atmosphere: oxygen (O2), nitrogen (N2) and argon (Ar); do not interact with infrared radiation significantly. While the oxygen and nitrogen molecules can vibrate, because of their symmetry these vibrations do not create any transient charge separation. Without such a transient dipole moment, they can neither absorb nor emit infrared radiation. In the Earth’s atmosphere, the dominant infrared absorbing gases are water vapor, carbon dioxide, and ozone (O3). The same molecules are also the dominant infrared emitting molecules. CO2 and O3 have "floppy" vibration motions whose quantum states can be excited by collisions at energies encountered in the atmosphere. For example, carbon dioxide is a linear molecule, but it has an important vibrational mode in which the molecule bends with the carbon in the middle moving one way and the oxygens on the ends moving the other way, creating some charge separation, a dipole moment, thus carbon dioxide molecules can absorb IR radiation. Collisions will immediately transfer this energy to heating the surrounding gas. On the other hand, other CO2 molecules will be vibrationally excited by collisions. Roughly 5% of CO2 molecules are vibrationally excited at room temperature and it is this 5% that radiates. A substantial part of the greenhouse effect due to carbon dioxide exists because this vibration is easily excited by infrared radiation. CO2 has two other vibrational modes. The symmetric stretch does not radiate, and the asymmetric stretch is at too high a frequency to be effectively excited by atmospheric temperature collisions, although it does contribute to absorption of IR radiation. The vibrational modes of water are at too high energies to effectively radiate, but do absorb higher frequency IR radiation. Water vapor has a bent shape. It has a permanent dipole moment (the O atom end is electron rich, and the H atoms electron poor) which means that IR radiation can be emitted and absorbed during rotational transitions, and these transitions can also be produced by collisional energy transfer. Clouds are also very important infrared absorbers. Therefore, water has multiple effects on infrared radiation, through its vapor phase and through its condensed phases. Other absorbers of significance include methane, nitrous oxide and the chlorofluorocarbons.
Discussion of the relative importance of different infrared absorbers is confused by the overlap between the spectral lines due to different gases, widened by pressure broadening. As a result, the absorption due to one gas cannot be thought of as independent of the presence of other gases. One convenient approach is to remove the chosen constituent, leaving all other absorbers, and the temperatures, untouched, and monitoring the infrared radiation escaping to space. The reduction in infrared absorption is then a measure of the importance of that constituent. More precisely, define the greenhouse effect (GE) to be the difference between the infrared radiation that the surface would radiate to space if there were no atmosphere and the actual infrared radiation escaping to space. Then compute the percentage reduction in GE when a constituent is removed. The table below is computed by this method, using a particular 1-dimensional model of the atmosphere. More recent 3D computations lead to similar results.
| Gas removed | percent reduction in GE |
|---|---|
| H2O | 36% |
| CO2 | 9% |
| O3 | 3% |
By this particular measure, water vapor can be thought of as providing 36% of the greenhouse effect, and carbon dioxide 9%, but the effect of removal of both of these constituents will be greater than the total that each reduces the effect, in this case, around 45%. An additional proviso is that these numbers are computed holding the cloud distribution fixed. But removing water vapor from the atmosphere while holding clouds fixed is not likely to be physically relevant. In addition, the effects of a given gas are typically nonlinear in the amount of that gas, since the absorption by the gas at one level in the atmosphere can remove photons that would otherwise interact with the gas at another altitude. The kinds of estimates presented in the table, while often encountered in the controversies surrounding global warming, must be treated with caution. Different estimates found in different sources typically result from different definitions and do not reflect uncertainties in the underlying radiative transfer.
Positive feedback and runaway greenhouse effect
When there is a loop of effects such as the concentration of a greenhouse gas itself being a function of temperature, there is a feedback. If the effect is to act in the same direction on temperature it is a positive feedback; and if in the opposite direction it is a negative feedback. Feedback effects can be on the same cause as the forcing, via another greenhouse gas, or on other effects such as change in ice cover affecting the planet's albedo.
Positive feedbacks do not have to lead to a runaway effect. With radiation from the Earth increasing in proportion to the fourth power of temperature, in accordance with the Stefan-Boltzmann law, the feedback effect has to be very strong to cause a runaway effect. An increase in temperature from greenhouse gases leading to increased water vapour which is a greenhouse gas causing further warming is a positive feedback. This cannot be a runaway effect or the runaway effect would have occurred long ago. Positive feedback effects are common and can always exist while runaway effects are much rarer and cannot be operating at all times.
If the effects from the second iteration of the loop of effects is larger than the effects of the first iteration of the loop this will lead to a self perpetuating effect. If this occurs and the feedback only ends after producing a major temperature increase, it is called a runaway greenhouse effect. A runaway feedback could also occur in the opposite direction leading to an ice age. Runaway feedbacks are bound to stop, since infinite temperatures are not observed. They are stopped by factors like a reducing supply of a greenhouse gas or a phase change of the gas or ice cover reducing towards zero or increasing toward a large size that is difficult to increase.
A runaway greenhouse effect involving CO2 and water vapor may have occurred on Venus. In this scenario, early Venus may have had a global ocean. As the brightness of the early sun increased, the amount of water vapor in the atmosphere increased, increasing the temperature and consequently increasing the evaporation of the ocean, leading eventually to the situation in which the oceans boiled, and all of the water vapor entered the atmosphere. On Venus today there is little water vapor in the atmosphere. If water vapor did contribute to the warmth of Venus at one time, this water is thought to have escaped to space. Venus is sufficiently strongly heated by the Sun that water vapor can rise much higher in the atmosphere and be split into hydrogen and oxygen by ultraviolet light. The hydrogen can then escape from the atmosphere and the oxygen recombines. Carbon dioxide, the dominant greenhouse gas in the current Venusian atmosphere, likely owes its larger concentration to the weakness of carbon recycling as compared to Earth, where the carbon dioxide emitted from volcanoes is efficiently subducted into the Earth by plate tectonics on geologic time scales.
According to the clathrate gun hypothesis a runaway greenhouse effect could be caused by liberation of methane gas from hydrates by global warming if there are sufficient hydrates close to unstable conditions. It has been speculated that the Permian-Triassic extinction event was caused by such a runaway effect. It is also thought that large quantities of methane could be released from the Siberian tundra as it begins to thaw, methane being 21 times more potent a greenhouse gas than carbon dioxide.
Anthropogenic greenhouse effect
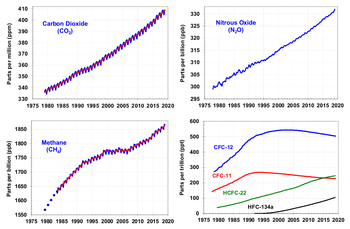
CO2 production from increased industrial activity (fossil fuel burning) and other human activities such as cement production and tropical deforestation has increased the CO2 concentrations in the atmosphere. Measurements of carbon dioxide amounts from Mauna Loa observatory show that CO2 has increased from about 313 ppm (parts per million) in 1960 to about 375 ppm in 2005. The current observed amount of CO2 exceeds the geological record of CO2 maxima (~300 ppm) from ice core data.
Because it is a greenhouse gas, elevated CO2 levels will increase global mean temperature; based on an extensive review of the scientific literature, the Intergovernmental Panel on Climate Change concludes that "most of the observed increase in globally averaged temperatures since the mid-20th century is very likely due to the observed increase in anthropogenic greenhouse gas concentrations".
Over the past 800,000 years, ice core data shows unambiguously that carbon dioxide has varied from values as low as 180 parts per million (ppm) to the pre-industrial level of 270ppm. Certain paleoclimatologists consider variations in carbon dioxide to be a fundamental factor in controlling climate variations over this time scale.
Real greenhouses
The term "greenhouse effect" can be a source of confusion as actual greenhouses do not function by the same mechanism the atmosphere does. Various materials at times imply incorrectly that they do, or do not make the distinction between the processes of radiation and convection.
The term 'greenhouse effect' originally came from the greenhouses used for gardening, but as mentioned the mechanism for greenhouses operates differently. Many sources make the "heat trapping" analogy of how a greenhouse limits convection to how the atmosphere performs a similar function through the different mechanism of infrared absorbing gases.
A greenhouse is usually built of glass, plastic, or a plastic-type material. It heats up mainly because the sun warms the ground inside it, which then warms the air in the greenhouse. The air continues to heat because it is confined within the greenhouse, unlike the environment outside the greenhouse where warm air near the surface rises and mixes with cooler air aloft. This can be demonstrated by opening a small window near the roof of a greenhouse: the temperature will drop considerably. It has also been demonstrated experimentally (Wood, 1909) that a "greenhouse" with a cover of rock salt heats up an enclosure similarly to one with a glass cover. Greenhouses thus work primarily by preventing convection; the atmospheric greenhouse effect however reduces radiation loss, not convection.
















No comments:
Post a Comment