Diabetes mellitus, often referred to simply as diabetes, is a syndrome of disordered metabolism, usually due to a combination of hereditary and environmental causes, resulting in abnormally high blood sugar levels (hyperglycemia). Blood glucose levels are controlled by a complex interaction of multiple chemicals and hormones in the body, including the hormone insulin made in the beta cells of the pancreas. Diabetes mellitus refers to the group of diseases that lead to high blood glucose levels due to defects in either insulin secretion or insulin action.
Diabetes develops due to a diminished production of insulin (in type 1) or resistance to its effects (in type 2 and gestational). Both lead to hyperglycaemia, which largely causes the acute signs of diabetes: excessive urine production, resulting compensatory thirst and increased fluid intake, blurred vision, unexplained weight loss, lethargy, and changes in energy metabolism. Monogenic forms, e.g. MODY, constitute 1-5 % of all cases.All forms of diabetes have been treatable since insulin became medically available in 1921, but there is no cure. The injections by a syringe or insulin pump deliver insulin, which is a basic treatment of type 1 diabetes. Type 2 is managed with a combination of dietary treatment, medications and insulin supplementation.
Diabetes and its treatments can cause many complications. Acute complications (hypoglycemia, ketoacidosis, or nonketotic hyperosmolar coma) may occur if the disease is not adequately controlled. Serious long-term complications include cardiovascular disease (doubled risk), chronic renal failure, retinal damage (which can lead to blindness), nerve damage, and microvascular damage, which may cause impotence and poor wound healing. Poor healing of wounds, particularly of the feet, can lead to gangrene, and possibly to amputation. Adequate treatment of diabetes, as well as increased emphasis on blood pressure control and lifestyle factors (such as not smoking and maintaining a healthy body weight), may improve the risk profile of most of the chronic complications. In the developed world, diabetes is the most significant cause of adult blindness in the non-elderly and the leading cause of non-traumatic amputation in adults, and diabetic nephropathy is the main illness requiring renal dialysis in the United States.Classification
The term diabetes, without qualification, usually refers to diabetes mellitus, which is associated with excessive sweet urine but there are several rarer conditions also named diabetes. The most common of these is diabetes insipidus in which the urine is not sweet; it can be caused by either kidney (nephrogenic DI) or pituitary gland (central DI) damage.
Most cases of diabetes mellitus fall into one of two broad categories. The term "type 1 diabetes" has universally replaced several former terms, including childhood-onset diabetes, juvenile diabetes, and insulin-dependent diabetes (IDDM). Likewise, the term "type 2 diabetes" has replaced several former terms, including adult-onset diabetes, obesity-related diabetes, and non-insulin-dependent diabetes (NIDDM). Various sources have defined "type 3 diabetes" as, among others, gestational diabetes, insulin-resistant type 1 diabetes (or "double diabetes"), type 2 diabetes which has progressed to require injected insulin, and latent autoimmune diabetes of adults. There is also maturity onset diabetes of the young (MODY) which is a group of several single gene (monogenic) disorders with strong family histories that present as type 2 diabetes.Type 1 diabetes mellitus
Type 1 diabetes mellitus is characterized by loss of the insulin-producing beta cells of the islets of Langerhans in the pancreas, leading to a deficiency of insulin. This type of diabetes can be further classified as immune-mediated or idiopathic. The majority of type 1 diabetes is of the immune-mediated variety, where beta cell loss is a T-cell mediated autoimmune attack. There is no known preventive measure which can be taken against type 1 diabetes; it is about 10% of diabetes mellitus cases in North America and Europe (though this varies by geographical location), and is a higher percentage in some other areas. Most affected people are otherwise healthy and of a healthy weight when onset occurs. Sensitivity and responsiveness to insulin are usually normal, especially in the early stages. Type 1 diabetes can affect children or adults but was traditionally termed "juvenile diabetes" because it represents a majority of the diabetes cases in children.
The principal treatment of type 1 diabetes, even in its earliest stages, is replacement of insulin combined with careful monitoring of blood glucose levels using blood testing monitors. Without insulin, diabetic ketoacidosis often develops which may result in coma or death. Treatment emphasis is now also placed on lifestyle adjustments (diet and exercise) though these cannot reverse the progress of the disease. Apart from the common subcutaneous injections, it is also possible to deliver insulin by a pump, which allows continuous infusion of insulin 24 hours a day at preset levels, and the ability to program doses (a bolus) of insulin as needed at meal times. Non-insulin treatments, such as monoclonal antibodies and stem-cell based therapies, are effective in animal models but have not yet completed clinical trials in humans.
Type 1 treatment must be continued indefinitely in essentially all cases. Treatment need not significantly impair normal activities, if sufficient patient training, awareness, appropriate care, discipline in testing and dosing of insulin is taken. However, treatment is burdensome for patients, insulin is replaced in a non-physiological manner, and this approach is therefore far from ideal. The average glucose level for the type 1 patient should be as close to normal (80–120 mg/dl, 4–6 mmol/l) as is safely possible. Some physicians suggest up to 140–150 mg/dl (7-7.5 mmol/l) for those having trouble with lower values, such as frequent hypoglycemic events. Values above 400 mg/dl (20 mmol/l) is sometimes accompanied by discomfort and frequent urination leading to dehydration. Values above 600 mg/dl (30 mmol/l) usually require medical treatment and may lead to ketoacidosis, although they are not immediately life-threatening. However, low levels of blood glucose, called hypoglycemia, may lead to seizures or episodes of unconsciousness and absolutely must be treated immediately.
Chronic insulin over treatment may result in Insulin resistance and obesity (type 2 diabetes) developing on top of type 1 which is analogous to the development of type 1 diabetes on top of type 2 when the beta cells are completely burned out as part of the natural history of the disease.
Type 2 diabetes mellitus
Type 2 diabetes mellitus is characterized differently due to insulin resistance or reduced insulin sensitivity, combined with relatively reduced, and sometimes absolute, insulin secretion. The defective responsiveness of body tissues to insulin almost certainly involves the insulin receptor in cell membranes. However, the specific defects are not known. Diabetes mellitus due to a known specific defect are classified separately.
In the early stage of type 2 diabetes, the predominant abnormality is reduced insulin sensitivity, characterized by elevated levels of insulin in the blood. At this stage hyperglycemia can be reversed by a variety of measures and medications that improve insulin sensitivity or reduce glucose production by the liver. As the disease progresses, the impairment of insulin secretion worsens, and therapeutic replacement of insulin often becomes necessary.
There are numerous theories as to the exact cause and mechanism in type 2 diabetes. Central obesity (fat concentrated around the waist in relation to abdominal organs, but not subcutaneous fat) is known to predispose individuals to insulin resistance. Abdominal fat is especially active hormonally, secreting a group of hormones called adipokines that may possibly impair glucose tolerance. Obesity is found in approximately 55% of patients diagnosed with type 2 diabetes. Other factors include aging and family history. In the last decade, type 2 diabetes has increasingly begun to affect children and adolescents, likely in connection with the increased prevalence of childhood obesity seen in recent decades in some places. Environmental exposures may contribute to recent increases in the rate of type 2 diabetes. A positive correlation has been found between the concentration in the urine of bisphenol A, a constituent of polycarbonate plastic, and the incidence of type 2 diabetes.
Type 2 diabetes may go unnoticed for years because visible symptoms are typically mild, non-existent or sporadic, and usually there are no ketoacidotic episodes. However, severe long-term complications can result from unnoticed type 2 diabetes, including renal failure due to diabetic nephropathy, vascular disease (including coronary artery disease), vision damage due to diabetic retinopathy, loss of sensation or pain due to diabetic neuropathy, and liver damage from non-alcoholic steatohepatitis.
Type 2 diabetes is usually first treated by increasing physical activity, decreasing carbohydrate intake, and losing weight. These can restore insulin sensitivity even when the weight loss is modest, for example around 5 kg (10 to 15 lb), most especially when it is in abdominal fat deposits. It is sometimes possible to achieve long-term, satisfactory glucose control with these measures alone. However, the underlying tendency to insulin resistance is not lost, and so attention to diet, exercise, and weight loss must continue. The usual next step, if necessary, is treatment with oral antidiabetic drugs. Insulin production is initially only moderately impaired in type 2 diabetes, so oral medication (often used in various combinations) can be used to improve insulin production (e.g., sulfonylureas), to regulate inappropriate release of glucose by the liver and attenuate insulin resistance to some extent (e.g., metformin), and to substantially attenuate insulin resistance (e.g., thiazolidinediones). According to one study, overweight patients treated with metformin compared with diet alone, had relative risk reductions of 32% for any diabetes endpoint, 42% for diabetes related death and 36% for all cause mortality and stroke. Oral medication may eventually fail due to further impairment of beta cell insulin secretion. At this point, insulin therapy is necessary to maintain normal or near normal glucose levels.
Gestational diabetes
Gestational diabetes mellitus (GDM) resembles type 2 diabetes in several respects, involving a combination of relatively inadequate insulin secretion and responsiveness. It occurs in about 2%–5% of all pregnancies and may improve or disappear after delivery. Gestational diabetes is fully treatable but requires careful medical supervision throughout the pregnancy. About 20%–50% of affected women develop type 2 diabetes later in life.
Even though it may be transient, untreated gestational diabetes can damage the health of the fetus or mother. Risks to the baby include macrosomia (high birth weight), congenital cardiac and central nervous system anomalies, and skeletal muscle malformations. Increased fetal insulin may inhibit fetal surfactant production and cause respiratory distress syndrome. Hyperbilirubinemia may result from red blood cell destruction. In severe cases, perinatal death may occur, most commonly as a result of poor placental profusion due to vascular impairment. Induction may be indicated with decreased placental function. A cesarean section may be performed if there is marked fetal distress or an increased risk of injury associated with macrosomia, such as shoulder dystocia.
A 2008 study completed in the U.S. found that more American women are entering pregnancy with preexisting diabetes. This is particularly problematic as diabetes raises the risk of complications during pregnancy, as well as increasing the potential that the children of diabetic mothers will also become diabetic in the future.
Other types
Most cases of diabetes mellitus fall into the two broad etiologic categories of type 1 or type 2 diabetes. However, many types of diabetes mellitus have known specific causes, and thus fall into separate categories as diabetes due to a specifc cause. As more research is done into diabetes, many patients who were previously diagnosed as type 1 or type 2 diabetes will be reclassified as diabetes due to their known specific cause.
Some cases of diabetes are caused by the body's tissue receptors not responding to insulin (even when insulin levels are normal, which is what separates it from type 2 diabetes); this form is very uncommon. Genetic mutations (autosomal or mitochondrial) can lead to defects in beta cell function. Abnormal insulin action may also have been genetically determined in some cases. Any disease that causes extensive damage to the pancreas may lead to diabetes (for example, chronic pancreatitis and cystic fibrosis). Diseases associated with excessive secretion of insulin-antagonistic hormones can cause diabetes (which is typically resolved once the hormone excess is removed). Many drugs impair insulin secretion and some toxins damage pancreatic beta cells.
Signs and symptoms
The classical triad of diabetes symptoms is polyuria, polydipsia and polyphagia, which are, respectively, frequent urination, increased thirst and consequent increased fluid intake, and increased appetite. Symptoms may develop quite rapidly (weeks or months) in type 1 diabetes, particularly in children. However, in type 2 diabetes symptoms usually develop much more slowly and may be subtle or completely absent. Type 1 diabetes may also cause a rapid yet significant weight loss (despite normal or even increased eating) and irreducible fatigue. All of these symptoms except weight loss can also manifest in type 2 diabetes in patients whose diabetes is poorly controlled.
When the glucose concentration in the blood is raised beyond its renal threshold, reabsorption of glucose in the proximal renal tubuli is incomplete, and part of the glucose remains in the urine (glycosuria). This increases the osmotic pressure of the urine and inhibits reabsorption of water by the kidney, resulting in increased urine production (polyuria) and increased fluid loss. Lost blood volume will be replaced osmotically from water held in body cells and other body compartments, causing dehydration and increased thirst.
Prolonged high blood glucose causes glucose absorption, which leads to changes in the shape of the lenses of the eyes, resulting in vision changes; sustained sensible glucose control usually returns the lens to its original shape. Blurred vision is a common complaint leading to a diabetes diagnosis; type 1 should always be suspected in cases of rapid vision change, whereas with type 2 change is generally more gradual, but should still be suspected.
Patients (usually with type 1 diabetes) may also initially present with diabetic ketoacidosis (DKA), an extreme state of metabolic dysregulation characterized by the smell of acetone on the patient's breath; a rapid, deep breathing known as Kussmaul breathing; polyuria; nausea; vomiting and abdominal pain; and any of many altered states of consciousness or arousal (such as hostility and mania or, equally, confusion and lethargy). In severe DKA, coma may follow, progressing to death. Diabetic ketoacidosis is a medical emergency and requires immediate hospitalization.
A rarer but equally severe possibility is hyperosmolar nonketotic state, which is more common in type 2 diabetes and is mainly the result of dehydration due to loss of body water. Often, the patient has been drinking extreme amounts of sugar-containing drinks, leading to a vicious circle in regard to the water loss.
Genetics
Both type 1 and type 2 diabetes are at least partly inherited. Type 1 diabetes appears to be triggered by some (mainly viral) infections, or less commonly, by stress or environmental exposure (such as exposure to certain chemicals or drugs). There is a genetic element in individual susceptibility to some of these triggers which has been traced to particular HLA genotypes (i.e., the genetic "self" identifiers relied upon by the immune system). However, even in those who have inherited the susceptibility, type 1 diabetes mellitus seems to require an environmental trigger. A small proportion of people with type 1 diabetes carry a mutated gene that causes maturity onset diabetes of the young (MODY).
There is a stronger inheritance pattern for type 2 diabetes. Those with first-degree relatives with type 2 have a much higher risk of developing type 2, increasing with the number of those relatives. Concordance among monozygotic twins is close to 100%, and about 25% of those with the disease have a family history of diabetes. Candidate genes include KCNJ11 (potassium inwardly rectifying channel, subfamily J, member 11), which encodes the islet ATP-sensitive potassium channel Kir6.2, and TCF7L2 (transcription factor 7–like 2), which regulates proglucagon gene expression and thus the production of glucagon-like peptide-1. Moreover, obesity (which is an independent risk factor for type 2 diabetes) is strongly inherited.
Various hereditary conditions may feature diabetes, for example myotonic dystrophy and Friedreich's ataxia. Wolfram's syndrome is an autosomal recessive neurodegenerative disorder that first becomes evident in childhood. It consists of diabetes insipidus, diabetes mellitus, optic atrophy, and deafness, hence the acronym DIDMOAD.
Pathophysiology

Insulin is the principal hormone that regulates uptake of glucose from the blood into most cells (primarily muscle and fat cells, but not central nervous system cells). Therefore deficiency of insulin or the insensitivity of its receptors plays a central role in all forms of diabetes mellitus.
Most of the carbohydrates in food are converted within a few hours to the monosaccharide glucose, the principal carbohydrate found in blood and used by the body as fuel. Insulin is released into the blood by beta cells (β-cells), found in the Islets of Langerhans in the pancreas, in response to rising levels of blood glucose after eating. Insulin is used by about two-thirds of the body's cells to absorb glucose from the blood for use as fuel, for conversion to other needed molecules, or for storage. Insulin is also the principal control signal for conversion of glucose to glycogen for internal storage in liver and muscle cells. Lowered glucose levels result both in the reduced release of insulin from the beta cells and in the reverse conversion of glycogen to glucose when glucose levels fall. This is mainly controlled by the hormone glucagon which acts in an opposite manner to insulin. Glucose thus recovered by the liver re-enters the bloodstream; muscle cells lack the necessary export mechanism.
Higher insulin levels increase some anabolic ("building up") processes such as cell growth and duplication, protein synthesis, and fat storage. Insulin (or its lack) is the principal signal in converting many of the bidirectional processes of metabolism from a catabolic to an anabolic direction, and vice versa. In particular, a low insulin level is the trigger for entering or leaving ketosis (the fat burning metabolic phase).
If the amount of insulin available is insufficient, if cells respond poorly to the effects of insulin (insulin insensitivity or resistance), or if the insulin itself is defective, then glucose will not be absorbed properly by those body cells that require it nor will it be stored appropriately in the liver and muscles. The net effect is persistent high levels of blood glucose, poor protein synthesis, and other metabolic derangements, such as acidosis.
Diagnosis
The diagnosis of type 1 diabetes, and many cases of type 2, is usually prompted by recent-onset symptoms of excessive urination (polyuria) and excessive thirst (polydipsia), often accompanied by weight loss. These symptoms typically worsen over days to weeks; about a quarter of people with new type 1 diabetes have developed some degree of diabetic ketoacidosis by the time the diabetes is recognized. The diagnosis of other types of diabetes is usually made in other ways. These include ordinary health screening; detection of hyperglycemia during other medical investigations; and secondary symptoms such as vision changes or unexplainable fatigue. Diabetes is often detected when a person suffers a problem that is frequently caused by diabetes, such as a heart attack, stroke, neuropathy, poor wound healing or a foot ulcer, certain eye problems, certain fungal infections, or delivering a baby with macrosomia or hypoglycemia.
Diabetes mellitus is characterized by recurrent or persistent hyperglycemia, and is diagnosed by demonstrating any one of the following:
- fasting plasma glucose level at or above 126 mg/dL (7.0 mmol/l).
- plasma glucose at or above 200 mg/dL (11.1 mmol/l) two hours after a 75 g oral glucose load as in a glucose tolerance test.
- symptoms of hyperglycemia and casual plasma glucose at or above 200 mg/dL (11.1 mmol/l).
A positive result, in the absence of unequivocal hyperglycemia, should be confirmed by a repeat of any of the above-listed methods on a different day. Most physicians prefer to measure a fasting glucose level because of the ease of measurement and the considerable time commitment of formal glucose tolerance testing, which takes two hours to complete. According to the current definition, two fasting glucose measurements above 126 mg/dL (7.0 mmol/l) is considered diagnostic for diabetes mellitus.
Patients with fasting glucose levels from 100 to 125 mg/dL (6.1 and 7.0 mmol/l) are considered to have impaired fasting glucose. Patients with plasma glucose at or above 140 mg/dL or 7.8 mmol/l, but not over 200, two hours after a 75 g oral glucose load are considered to have impaired glucose tolerance. Of these two pre-diabetic states, the latter in particular is a major risk factor for progression to full-blown diabetes mellitus as well as cardiovascular disease.
While not used for diagnosis, an elevated level of glucose irreversibly bound to hemoglobin (termed glycosylated hemoglobin or HbA1c) of 6.0% or higher is considered abnormal by most labs; HbA1c is primarily used as a treatment-tracking test reflecting average blood glucose levels over the preceding 90 days (approximately). However, some physicians may order this test at the time of diagnosis to track changes over time. The current recommended goal for HbA1c in patients with diabetes is <7.0%,>
Screening
Diabetes screening is recommended for many people at various stages of life, and for those with any of several risk factors. The screening test varies according to circumstances and local policy, and may be a random blood glucose test, a fasting blood glucose test, a blood glucose test two hours after 75 g of glucose, or an even more formal glucose tolerance test. Many healthcare providers recommend universal screening for adults at age 40 or 50, and often periodically thereafter. Earlier screening is typically recommended for those with risk factors such as obesity, family history of diabetes, high-risk ethnicity.
Many medical conditions are associated with diabetes and warrant screening. A partial list includes: high blood pressure, elevated cholesterol levels, coronary artery disease, past gestational diabetes, polycystic ovary syndrome, chronic pancreatitis, fatty liver, hemochromatosis, cystic fibrosis, several mitochondrial neuropathies and myopathies, myotonic dystrophy, Friedreich's ataxia, some of the inherited forms of neonatal hyperinsulinism. The risk of diabetes is higher with chronic use of several medications, including high-dose glucocorticoids, some chemotherapy agents (especially L-asparaginase), as well as some of the antipsychotics and mood stabilizers (especially phenothiazines and some atypical antipsychotics).
People with a confirmed diagnosis of diabetes are tested routinely for complications. This includes yearly urine testing for microalbuminuria and examination of the retina of the eye for retinopathy. In the UK, systematic screening for diabetic retinopathy, which can be effectively treated if detected at an early stage, has helped reduce visual impairment in people with diabetes since its implementation.
Prevention
Type 1 diabetes risk is known to depend upon a genetic predisposition based on HLA types (particularly types DR3 and DR4), an unknown environmental trigger (suspected to be an infection, although none has proven definitive in all cases), and an uncontrolled autoimmune response that attacks the insulin producing beta cells. Some research has suggested that breastfeeding decreased the risk in later life; various other nutritional risk factors are being studied, but no firm evidence has been found. Giving children 2000 IU of Vitamin D during their first year of life is associated with reduced risk of type 1 diabetes, though the causal relationship is obscure.
Children with antibodies to beta cell proteins (ie, at early stages of an immune reaction to them) but no overt diabetes, and treated with vitamin B-3 (niacin), had less than half the diabetes onset incidence in a 7-year time span as did the general population, and an even lower incidence relative to those with antibodies as above, but who received no vitamin B3.
Type 2 diabetes risk can be reduced in many cases by making changes in diet and increasing physical activity. The American Diabetes Association (ADA) recommends maintaining a healthy weight, getting at least 2½ hours of exercise per week (several brisk sustained walks appear sufficient), having a modest fat intake, and eating sufficient fiber (eg, from whole grains). The ADA does not recommend alcohol consumption as a preventive, but it is interesting to note that moderate alcohol intake may reduce the risk (though heavy consumption absolutely and clearly increases damage to bodily systems significantly); a similarly confused connection between low dose alcohol consumption and heart disease is termed the French Paradox.
There is inadequate evidence that eating foods of low glycemic index is clinically helpful despite recommendations and suggested diets emphasizing this approach.
There are numerous studies which suggest connections between some aspects of Type II diabetes with ingestion of certain foods or with some drugs. Some studies have shown delayed progression to diabetes in predisposed patients through prophylactic use of metformin, rosiglitazone, or valsartan. In patients on hydroxychloroquine for rheumatoid arthritis, incidence of diabetes was reduced by 77% though causal mechanisms are unclear. Breastfeeding may also be associated with the prevention of type 2 of the disease in mothers. Clear evidence for these and any of many other connections between foods and supplements and diabetes is sparse to date; none, despite secondary claims for (or against), is sufficiently well established to justify as a standard clinical approach.
Treatment and management
Diabetes mellitus is currently a chronic disease, without a cure, and medical emphasis must necessarily be on managing/avoiding possible short-term as well as long-term diabetes-related problems. There is an exceptionally important role for patient education, dietetic support, sensible exercise, self monitoring of blood glucose, with the goal of keeping both short-term blood glucose levels, and long term levels as well, within acceptable bounds. Careful control is needed to reduce the risk of long term complications. This is theoretically achievable with combinations of diet, exercise and weight loss (type 2), various oral diabetic drugs (type 2 only), and insulin use (type 1 and for type 2 not responding to oral medications, mostly those with extended duration diabetes). In addition, given the associated higher risks of cardiovascular disease, lifestyle modifications should be undertaken to control blood pressure and cholesterol by exercising more, smoking less or ideally not at all, consuming an appropriate diet, wearing diabetic socks, wearing diabetic shoes, and if necessary, taking any of several drugs to reduce blood pressure. Many Type 1 treatments include combination use of regular or NPH insulin, and/or synthetic insulin analogs (eg, Humalog, Novolog or Apidra) in combinations such as Lantus/Levemir and Humalog, Novolog or Apidra. Another Type 1 treatment option is the use of the insulin pump (eg, from Deltec Cozmo, Animas, Medtronic Minimed, Insulet Omnipod, or ACCU-CHEK). A blood lancet is used to pierce the skin (typically of a finger), in order to draw blood to test it for sugar levels.
In countries using a general practitioner system, such as the United Kingdom, care may take place mainly outside hospitals, with hospital-based specialist care used only in case of complications, difficult blood sugar control, or research projects. In other circumstances, general practitioners and specialists share care of a patient in a team approach. Optometrists, podiatrists/chiropodists, dietitians, physiotherapists, nursing specialists (eg, DSNs (Diabetic Specialist Nurse)), nurse practitioners, or Certified Diabetes Educators, may jointly provide multidisciplinary expertise. In addition to the medications and supplies needed, patients are often advised to receive regular consultation from a physician (e.g., at least every three to six months).
Prognosis
Patient education, understanding, and participation is vital since the complications of diabetes are far less common and less severe in people who have well-controlled blood sugar levels. Wider health problems accelerate the deleterious effects of diabetes. These include smoking, elevated cholesterol levels, obesity, high blood pressure, and lack of regular exercise. According to a study, women with high blood pressure have a threefold risk of developing diabetes.
Anecdotal evidence suggests that some of those with type 2 diabetes who exercise regularly, lose weight, and eat healthy diets may be able to keep some of the disease or some of the effects of the disease in 'remission.' Certainly these tips can help prevent people predisposed to type 2 diabetes and those at pre-diabetic stages from actually developing the disorder as it helps restore insulin sensitivity. However patients should talk to their doctors about this for real expectations before undertaking it (esp. to avoid hypoglycemia or other complications); few people actually seem to go into total 'remission,' but some may find they need less of their insulin medications since the body tends to have lower insulin requirements during and shortly following exercise. Regardless of whether it works that way or not for an individual, there are certainly other benefits to this healthy lifestyle for both diabetics and nondiabetics.
The way diabetes is managed changes with age. Insulin production decreases because of age-related impairment of pancreatic beta cells. Additionally, insulin resistance increases because of the loss of lean tissue and the accumulation of fat, particularly intra-abdominal fat, and the decreased tissue sensitivity to insulin. Glucose tolerance progressively declines with age, leading to a high prevalence of type 2 diabetes and postchallenge hyperglycemia in the older population. Age-related glucose intolerance in humans is often accompanied by insulin resistance, but circulating insulin levels are similar to those of younger people. Treatment goals for older patients with diabetes vary with the individual, and take into account health status, as well as life expectancy, level of dependence, and willingness to adhere to a treatment regimen.
Acute complications
- Diabetic ketoacidosis
Diabetic ketoacidosis (DKA) is an acute and dangerous complication that is always a medical emergency. Low insulin levels cause the liver to turn to fat for fuel (ie, ketosis); ketone bodies are intermediate substrates in that metabolic sequence. This is normal when periodic, but can become a serious problem if sustained. Elevated levels of ketone bodies in the blood decrease the blood's pH, leading to DKA. On presentation at hospital, the patient in DKA is typically dehydrated, and breathing rapidly and deeply. Abdominal pain is common and may be severe. The level of consciousness is typically normal until late in the process, when lethargy may progress to coma. Ketoacidosis can easily become severe enough to cause hypotension, shock, and death. Urine analysis will reveal significant levels of ketone bodies (which have exceeded their renal threshold blood levels to appear in the urine, often before other overt symptoms). Prompt, proper treatment usually results in full recovery, though death can result from inadequate or delayed treatment, or from complications (dg, brain edema). DKA is always a medical emergency and requires medical attention. Ketoacidosis is much more common in type 1 diabetes than type 2.
- Nonketotic hyperosmolar coma
Hyperosmolar nonketotic state (HNS) is an acute complication sharing many symptoms with DKA, but an entirely different origin and different treatment. A person with very high (usually considered to be above 300 mg/dl (16 mmol/l)) blood glucose levels, water is osmotically drawn out of cells into the blood and the kidneys eventually begin to dump glucose into the urine. This results in loss of water and an increase in blood osmolarity. If fluid is not replaced (by mouth or intravenously), the osmotic effect of high glucose levels, combined with the loss of water, will eventually lead to dehydration. The body's cells become progressively dehydrated as water is taken from them and excreted. Electrolyte imbalances are also common and are always dangerous. As with DKA, urgent medical treatment is necessary, commonly beginning with fluid volume replacement. Lethargy may ultimately progress to a coma, though this is more common in type 2 diabetes than type 1.
- Hypoglycemia
Hypoglycemia, or abnormally low blood glucose, is an acute complication of several diabetes treatments. It is rare otherwise, either in diabetic or non-diabetic patients. The patient may become agitated, sweaty, and have many symptoms of sympathetic activation of the autonomic nervous system resulting in feelings akin to dread and immobilized panic. Consciousness can be altered or even lost in extreme cases, leading to coma, seizures, or even brain damage and death. In patients with diabetes, this may be caused by several factors, such as too much or incorrectly timed insulin, too much or incorrectly timed exercise (exercise decreases insulin requirements) or not enough food (specifically glucose containing carbohydrates). The variety of interactions makes cause identification difficult in many instances.
It is more accurate to note that iatrogenic hypoglycemia is typically the result of the interplay of absolute (or relative) insulin excess and compromised glucose counterregulation in type 1 and advanced type 2 diabetes. Decrements in insulin, increments in glucagon, and, absent the latter, increments in epinephrine are the primary glucose counterregulatory factors that normally prevent or (more or less rapidly) correct hypoglycemia. In insulin-deficient diabetes (exogenous) insulin levels do not decrease as glucose levels fall, and the combination of deficient glucagon and epinephrine responses causes defective glucose counterregulation.
Furthermore, reduced sympathoadrenal responses can cause hypoglycemia unawareness. The concept of hypoglycemia-associated autonomic failure (HAAF) in diabetes posits that recent incidents of hypoglycemia causes both defective glucose counterregulation and hypoglycemia unawareness. By shifting glycemic thresholds for the sympathoadrenal (including epinephrine) and the resulting neurogenic responses to lower plasma glucose concentrations, antecedent hypoglycemia leads to a vicious cycle of recurrent hypoglycemia and further impairment of glucose counterregulation. In many cases (but not all), short-term avoidance of hypoglycemia reverses hypoglycemia unawareness in affected patients, although this is easier in theory than in clinical experience.
In most cases, hypoglycemia is treated with sugary drinks or food. In severe cases, an injection of glucagon (a hormone with the opposite effects of insulin) or an intravenous infusion of dextrose is used for treatment, but usually only if the person is unconscious. In any given incident, glucogon will only work once as it uses stored liver glycogen as a glucose source; in the absence of such stores, glucagon is largely ineffective. In hospitals, intravenous dextrose is often used.
Chronic complications
- Vascular disease
Chronic elevation of blood glucose level leads to damage of blood vessels (angiopathy). The endothelial cells lining the blood vessels take in more glucose than normal, since they don't depend on insulin. They then form more surface glycoproteins than normal, and cause the basement membrane to grow thicker and weaker. In diabetes, the resulting problems are grouped under "microvascular disease" (due to damage to small blood vessels) and "macrovascular disease" (due to damage to the arteries).
The damage to small blood vessels leads to a microangiopathy, which can cause one or more of the following:
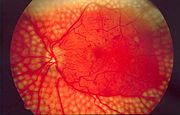
- Diabetic retinopathy, growth of friable and poor-quality new blood vessels in the retina as well as macular edema (swelling of the macula), which can lead to severe vision loss or blindness. Retinal damage (from microangiopathy) makes it the most common cause of blindness among non-elderly adults in the US.
- Diabetic neuropathy, abnormal and decreased sensation, usually in a 'glove and stocking' distribution starting with the feet but potentially in other nerves, later often fingers and hands. When combined with damaged blood vessels this can lead to diabetic foot (see below). Other forms of diabetic neuropathy may present as mononeuritis or autonomic neuropathy. Diabetic amyotrophy is muscle weakness due to neuropathy.
- Diabetic nephropathy, damage to the kidney which can lead to chronic renal failure, eventually requiring dialysis. Diabetes mellitus is the most common cause of adult kidney failure worldwide in the developed world.
- Diabetic cardiomyopathy, damage to the heart, leading to diastolic dysfunction and eventually heart failure.
Macrovascular disease leads to cardiovascular disease, to which accelerated atherosclerosis is a contributor:
- Coronary artery disease, leading to angina or myocardial infarction ("heart attack")
- Stroke (mainly the ischemic type)
- Peripheral vascular disease, which contributes to intermittent claudication (exertion-related leg and foot pain) as well as diabetic foot.
- Diabetic myonecrosis ('muscle wasting')
Diabetic foot, often due to a combination of sensory neuropathy (numbness or insensitivity) and vascular damage, increase rates of skin ulcers and infection and, in serious cases, necrosis and gangrene. It is why diabetics are prone to leg and foot infections and why it takes longer for them to heal from leg and foot wounds. It is the most common cause of non-traumatic adult amputation, usually of toes and or feet, in the developed world.
Carotid artery stenosis does not occur more often in diabetes, and there appears to be a lower prevalence of abdominal aortic aneurysm. However, diabetes does cause higher morbidity, mortality and operative risks with these conditions.
Diabetic encephalopathy is the increased cognitive decline and risk of dementia observed in diabetes. Various mechanisms are proposed, including alterations to the vascular supply of the brain and the interaction of insulin with the brain itself.











No comments:
Post a Comment