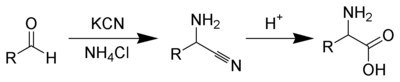
The Strecker amino acid synthesis, devised by Adolph Strecker, is a series of chemical reactions that synthesize an amino acid from an aldehyde (or ketone). The aldehyde is condensed with ammonium chloride in the presence of potassium cyanide to form an α-aminonitrile, which is subsequently hydrolyzed to give the desired amino-acid.
While usage of ammonium salts gives unsubstituted amino acids, primary and secondary amines also successfully give substituted amino acids. Likewise, the usage of ketones, instead of aldehydes, gives α,α-disubstituted amino acids.
The traditional synthesis of Adolph Strecker from 1850 gives racemic α-amino nitriles, but recently several procedures utilizing asymmetric auxiliaries or asymmetric catalysts have been developed.Reaction mechanism
The reaction mechanism for this reaction is sketched below. In part one aldehyde 1.1 reacts with ammonia in a nucleophilic addition to the hemiaminal 1.3 which attracts a proton to form iminium ion 1.5 by elimination of water. A second nucleophilic addition of the cyanide ion forms the aminonitrile 1.6.
 |  | |
| Strecker synthesis part I | Strecker synthesis part II |
In stage two a proton activates aminonitrile 2.1 for nucleophilic addition of two equivalents of water to intermediate 2.6 which eliminates ammonia and a proton to final product 2.7.
Scope
An example of present-day use of the Strecker synthesis is a multikilogram scale synthesis of a valine derivative starting from 3-methyl-2-butanone:












No comments:
Post a Comment